1. Identify the condensed formula of the given compound from the following.
ClCH2CH2CH2CH2CHCH2CH2CH2CHCH2CH3
a) Cl (CH2)2 (CH2)2CH (CH2)2CH2CH2CH3
b) Cl (CH2)3CH2CHCH2CH2CH2CH (CH3) 2
c) Cl (CH2)4CH (CH2)3CH (CH2) CH3
d) Cl (CH2)3CH2CH2CH (CH3)2CH3CHCH2CH3
Answer: c
Explanation: To write the condensed formula, group together all the repeating CH₂ units and ensure that each carbon forms exactly four bonds. In the given structure ClCH₂CH₂CH₂CH₂CHCH₂CH₂CH₂CHCH₂CH₃, there are four consecutive CH₂ groups, which can be written as (CH₂)₄. Applying the same method to the rest of the chain and combining the CH₂ units accordingly, we arrive at the condensed formula of the molecule.
2. Identify the condensed formula of ethane from the following.
a) CH3-CH3
b) HC-H2-C-H3
c) CH2=CH2
d) CH3-CH2
Answer: a
Explanation: In a condensed formula, all atoms are written out, but single bonds are usually not shown. However, double and triple bonds are represented. The formula CH₂=CH₂ does not represent ethane; it represents ethene.
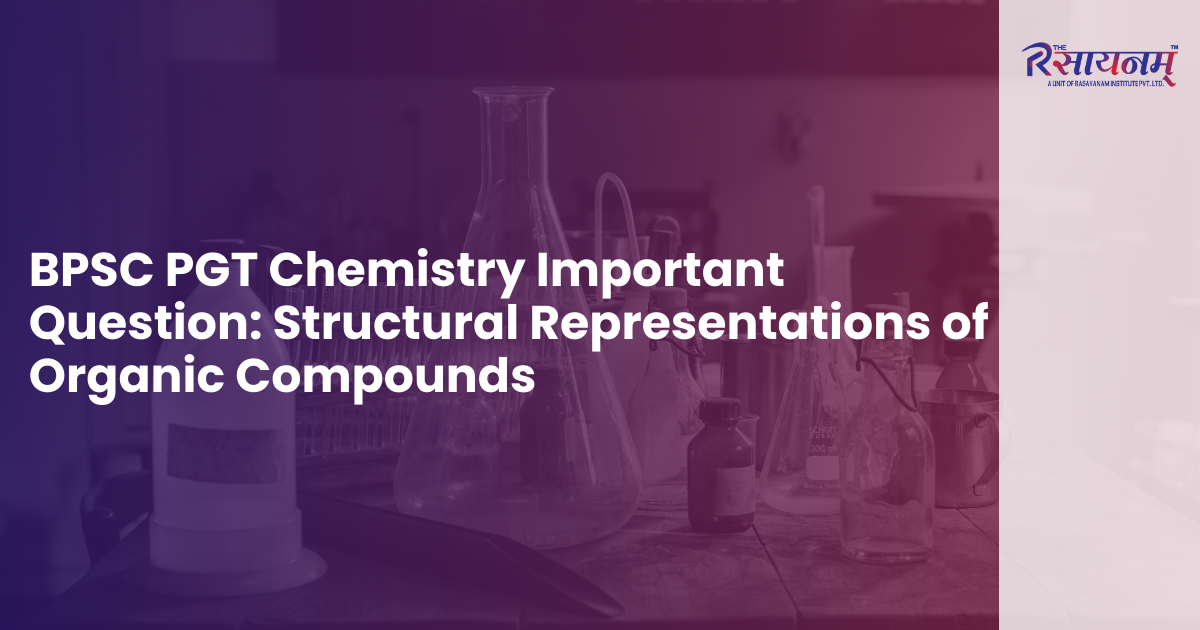
3. Determine the bond-line formula of CH3CH2COCH2CH3.
a) 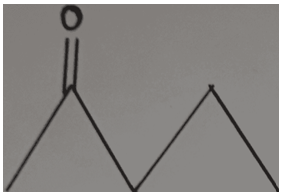
b) 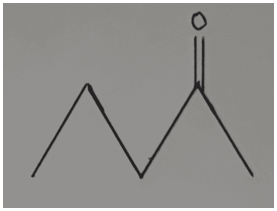
c) 
d) 
Answer: c
Explanation: In the compound CH₃CH₂COCH₂CH₃, the ketone group (C=O) is bonded to the third carbon atom. Therefore, the double bond must be shown at the position of the third carbon, that is, at the end of the second line. In all the other options, the ketone group is not located on the third carbon, which makes them incorrect.
4. Which is not a heteroatom?
a) Oxygen
b) Nitrogen
c) Carbon
d) Sulphur
Answer: c
Explanation: A heteroatom is any atom in an organic compound that is not carbon or hydrogen. Thus, all atoms other than carbon and hydrogen in the given options are considered heteroatoms.
a) True
b) False
Explanation: In a bond-line (skeletal) representation, only carbon and hydrogen atoms are not explicitly shown, while other atoms such as oxygen, nitrogen, chlorine, etc. are always written. The bonds are represented by lines according to their bond order—one line for a single bond, two lines for a double bond, and three lines for a triple bond.
Related Question Bank to Read:
BPSC PGT Chemistry: p-Block Elements Important Questions
BPSC PGT Chemistry Important Question: Uses of Boron and Aluminium and their Compounds
BPSC PGT Chemistry: Trends and Anomalous Properties of Boron Important Questions
BPSC PGT Chemistry: p-Block Elements Important Questions
Silicon & P-Block Elements: Understanding Their Properties and Applications
Boric Acid & P-Block Element: Understanding Their Properties and Applications
BPSC PGT Chemistry: Important Compounds of Boron
 Download The Rasayanam App
Download The Rasayanam App

Also, download our brochure for more details on the program and contact us with any queries.
Conclusion
If you’re preparing for BPSC PGT Chemistry, The Rasayanam provides structured courses, expert mentorship, and top-quality study resources to help you excel.


